Freeze-drying
Freeze-drying (also known as lyophilization or cryodesiccation) is a dehydration process typically used to preserve a perishable material or make the material more convenient for transport. Freeze-drying works by freezing the material and then reducing the surrounding pressure and adding enough heat to allow the frozen water in the material to sublime directly from the solid phase to the gas phase.
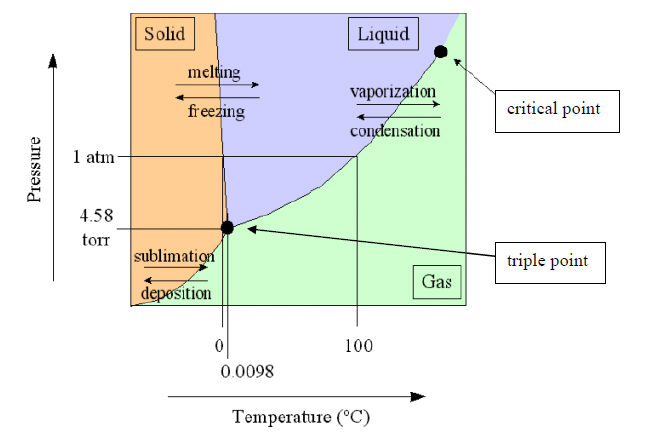
As shown below on the phase diagram for water, low pressures are required for sublimation to take place[1]:
Contents |
The origins of freeze drying
Freeze-drying was first actively developed during WWII. Serum being sent to Europe for medical treatment of the wounded required refrigeration. Due to the lack of available refrigeration, many serum supplies were spoiling before reaching the intended recipients. The freeze-drying process was developed as a commercial technique that enabled serum to be rendered chemically stable and viable without having to be refrigerated. Shortly thereafter, the freeze dry process was applied to penicillin and bone, and lyophilization became recognized as an important scientific technique for preservation of biologicals. Since that time, freeze-drying has been used as a preservation or processing technique for a wide variety of products. Some of the applications include the processing of pharmaceuticals, diagnostic kits, restoration of water damaged documents, river bottom sludge prepared for hydrocarbon analysis, ceramics used in the semiconductor industry, viral or bacterial cultures, tissues prepared for analysis, the production of synthetic skins and restoration of historic/reclaimed boat hulls.[2]
The freeze-drying process
There are four stages in the complete drying process: pretreatment, freezing, primary drying, and secondary drying.
Pretreatment
Pretreatment includes any method of treating the product prior to freezing. This may include concentrating the product, formulation revision (i.e., addition of components to increase stability and/or improve processing), decreasing a high vapor pressure solvent or increasing the surface area. In many instances the decision to pretreat a product is based on theoretical knowledge of freeze-drying and its requirements, or is demanded by cycle time or product quality considerations. Methods of pretreatment include: Freeze concentration, Solution phase concentration, Formulation to Preserve Product Appearance, Formulation to Stabilize Reactive Products, Formulation to Increase the Surface Area, and Decreasing High Vapor Pressure Solvents.[2]
Freezing
In a lab, this is often done by placing the material in a freeze-drying flask and rotating the flask in a bath, called a shell freezer, which is cooled by mechanical refrigeration, dry ice and methanol, or liquid nitrogen. On a larger scale, freezing is usually done using a freeze-drying machine. In this step, it is important to cool the material below its triple point, the lowest temperature at which the solid and liquid phases of the material can coexist. This ensures that sublimation rather than melting will occur in the following steps. Larger crystals are easier to freeze-dry. To produce larger crystals, the product should be frozen slowly or can be cycled up and down in temperature. This cycling process is called annealing. However, in the case of food, or objects with formerly-living cells, large ice crystals will break the cell walls (a problem discovered, and solved, by Clarence Birdseye), resulting in the destruction of more cells, which can result in increasingly poor texture and nutritive content. In this case, the freezing is done rapidly, in order to lower the material to below its eutectic point quickly, thus avoiding the formation of ice crystals. Usually, the freezing temperatures are between −50 °C and −80 °C. The freezing phase is the most critical in the whole freeze-drying process, because the product can be spoiled if badly done.
Amorphous materials do not have a eutectic point, but they do have a critical point, below which the product must be maintained to prevent melt-back or collapse during primary and secondary drying.
Primary drying
During the primary drying phase, the pressure is lowered (to the range of a few millibars), and enough heat is supplied to the material for the water to sublimate. The amount of heat necessary can be calculated using the sublimating molecules’ latent heat of sublimation. In this initial drying phase, about 95% of the water in the material is sublimated. This phase may be slow (can be several days in the industry), because, if too much heat is added, the material’s structure could be altered.
In this phase, pressure is controlled through the application of partial vacuum. The vacuum speeds sublimation, making it useful as a deliberate drying process. Furthermore, a cold condenser chamber and/or condenser plates provide a surface(s) for the water vapour to re-solidify on. This condenser plays no role in keeping the material frozen; rather, it prevents water vapor from reaching the vacuum pump, which could degrade the pump's performance. Condenser temperatures are typically below −50 °C (−60 °F).
It is important to note that, in this range of pressure, the heat is brought mainly by conduction or radiation; the convection effect is considered to be inefficient.
Secondary drying
The secondary drying phase aims to remove unfrozen water molecules, since the ice was removed in the primary drying phase. This part of the freeze-drying process is governed by the material’s adsorption isotherms. In this phase, the temperature is raised higher than in the primary drying phase, and can even be above 0 °C, to break any physico-chemical interactions that have formed between the water molecules and the frozen material. Usually the pressure is also lowered in this stage to encourage desorption (typically in the range of microbars, or fractions of a pascal). However, there are products that benefit from increased pressure as well.
After the freeze-drying process is complete, the vacuum is usually broken with an inert gas, such as nitrogen, before the material is sealed.
At the end of the operation, the final residual water content in the product is extremely low, around 1% to 4%.
Properties of freeze-dried products
If a freeze-dried substance is sealed to prevent the reabsorption of moisture, the substance may be stored at room temperature without refrigeration, and be protected against spoilage for many years. Preservation is possible because the greatly reduced water content inhibits the action of microorganisms and enzymes that would normally spoil or degrade the substance.
Freeze-drying also causes less damage to the substance than other dehydration methods using higher temperatures. Freeze-drying does not usually cause shrinkage or toughening of the material being dried. In addition, flavours, smells and nutritional content generally remain unchanged, making the process popular for preserving food[3]. However, water is not the only chemical capable of sublimation, and the loss of other volatile compounds such as acetic acid (vinegar) and alcohols can yield undesirable results.
Freeze-dried products can be rehydrated (reconstituted) much more quickly and easily because the process leaves microscopic pores. The pores are created by the ice crystals that sublimate, leaving gaps or pores in their place. This is especially important when it comes to pharmaceutical uses. Freeze-drying can also be used to increase the shelf life of some pharmaceuticals for many years.
Freeze-drying protectants
Similar to cryoprotectants, some molecules protect freeze-dried material. Known as lyoprotectants, these molecules are typically polyhydroxy compounds such as sugars (mono-, di-, and polysaccharides), polyalcohols, and their derivatives. Trehalose and sucrose are natural lyoprotectants. Trehalose is produced by a variety of plant, fungi, and invertebrate animals that remain in a state of suspended animation during periods of drought (also known as anhydrobiosis).
Applications of freeze-drying
Pharmaceutical and biotechnology
Pharmaceutical companies often use freeze-drying to increase the shelf life of products, such as vaccines and other injectables. By removing the water from the material and sealing the material in a vial, the material can be easily stored, shipped, and later reconstituted to its original form for injection.
Food industry

Freeze-drying is used to preserve food and make it very lightweight. The process has been popularized in the forms of freeze-dried ice cream, an example of astronaut food. It is also popular and convenient for hikers because the reduced weight allows them to carry more food and reconstitute it with available water. Instant coffee is sometimes freeze-dried, despite the high costs of the freeze-driers used. The coffee is often dried by vaporization in a hot air flow, or by projection on hot metallic plates. Freeze-dried fruit is used in some breakfast cereal. Culinary herbs are also freeze-dried, although air-dried herbs are far more common and less expensive. However, the freeze-drying process is used more commonly in the pharmaceutical industry.
Technological industry
In chemical synthesis, products are often freeze-dried to make them more stable, or easier to dissolve in water for subsequent use.
In bioseparations, freeze-drying can be used also as a late-stage purification procedure, because it can effectively remove solvents. Furthermore, it is capable of concentrating substances with low molecular weights that are too small to be removed by a filtration membrane.
Freeze-drying is a relatively expensive process. The equipment is about three times as expensive as the equipment used for other separation processes, and the high energy demands lead to high energy costs. Furthermore, freeze-drying also has a long process time, because the addition of too much heat to the material can cause melting or structural deformations. Therefore, freeze-drying is often reserved for materials that are heat-sensitive, such as proteins, enzymes, microorganisms, and blood plasma. The low operating temperature of the process leads to minimal damage of these heat-sensitive products
Other uses
Organizations such as the Document Conservation Laboratory at the United States National Archives and Records Administration (NARA) have done studies on freeze-drying as a recovery method of water-damaged books and documents. While recovery is possible, restoration quality depends on the material of the documents. If a document is made of a variety of materials, which have different absorption properties, expansion will occur at a non-uniform rate, which could lead to deformations. Water can also cause mold to grow or make inks bleed. In these cases, freeze-drying may not be an effective restoration method.
In bacteriology freeze-drying is used to conserve special strains.
In high-altitude environments, the low temperatures and pressures can sometimes produce natural mummies by a process of freeze-drying.
Advanced ceramics processes sometimes use freeze-drying to create a formable powder from a sprayed slurry mist. Freeze-drying creates softer particles with a more homogeneous chemical composition than traditional hot spray drying, but it is also more expensive.
Freeze drying is also used for floral preservation. Wedding bouquet preservation has become very popular with brides who want to preserve their wedding day flowers [4]
Freeze-drying equipment
Rotary freeze-driers are usually used with liquid products, such as pharmaceutical solutions and tissue extracts.

Manifold freeze-driers are usually used when drying a large amount of small containers and the product will be used in a short period of time. A manifold drier will dry the product to less than 5% moisture content. Without heat, only primary drying (removal of the unbound water) can be achieved. A heater must be added for secondary drying, which will remove the bound water and will produce a lower moisture content.

Tray freeze-driers are more sophisticated and are used to dry a variety of materials. A tray freeze-drier is used to produce the driest product for long-term storage. A tray freeze-drier allows the product to be frozen in place and performs both primary (unbound water removal) and secondary (bound water removal) freeze-drying, thus producing the driest possible end-product. Tray freeze-driers can dry products in bulk or in vials. When drying in vials, the freeze-drier is supplied with a stoppering mechanism that allows a stopper to be pressed into place, sealing the vial before it is exposed to the atmosphere. This is used for long-term storage, such as vaccines.
Improved freeze drying techniques are being developed to extend the range of products that can be freeze dried, to improve the quality of the product, and to produce the product faster with less labor.
Ever since the 1930s, industrial freeze drying had been dependent on a single type of equipment: the tray freeze drier. In 2005 a quicker and less-labor intensive freeze drying method was developed for bulk materials. This freeze drying process proved to be able to produce free-flowing powder from a single vessel. Known as [Active Freeze Drying] AFD technology, the new process used continuous motion to improve mass transfer and hence cutting processing time, while also eliminating the need to transfer to and from drying trays and downstream size reduction devices.

There are essentially three categories of freeze-driers: the rotary evaporator freeze-drier, the manifold freeze-drier, and the tray freeze-drier.
See also
- Food preservation
- Supercritical drying
- Freeze-dried Food and NASA
References
- ↑ Barley, J. (2009) "Basic Principles of Freeze Drying"
- ↑ 2.0 2.1 Dr. Pikal, M. and Reiter, C. (2008) "Basic Theory of Freeze Drying"
- ↑ "Freeze Dried & Dehydrated Food Explained". Packitgourmet.com. http://www.packitgourmet.com/Freeze-Dried+%26amp%3B+Dehydrated+Explained-sp67.html. Retrieved 2010-06-23.
- ↑ Brian Donaldson. "Wedding Bouquet Preservation…saver your special memories of your wedding day through the preservation of your bridal bouquet!" (MHT). http://www.mountainviewfreezedry.com/Wedding%20Bouquet%20Preservation%20article.mht. Retrieved 2010-06-23.
- Harris, E. L. V. and S. Angal (1989). Protein Purification Methods. Oxford University Press. ISBN 0-19-963003-8
- Kennedy, John F. and Joaquim M. S. Cabral (1993). Recovery Processes for Biological Materials. John Wiley & Sons Ltd.
- Efficacy of Various Drying Methods
External links
- International Society of Lyophilization - Freeze Drying
- Freeze Drying Webinars presented by Industry Experts
- Freeze Drying Technical Briefs in Multiple Languages
- Freeze Drying Info Website
- Freeze-drying vs. Dehydration
- Pharmaceutical research, new technical developments, PAT, newest Publications Research group on freeze-drying of university of Erlangen, Germany (engl. Website)
- FDA Guide to inspections of lyophilization of parentals
- [http://www.rpi.edu/dept/chem-eng/Biotech-Environ/LYO/index.html Lyophilization: Freeze-Drying
A Downstream Process]
|
|||||